
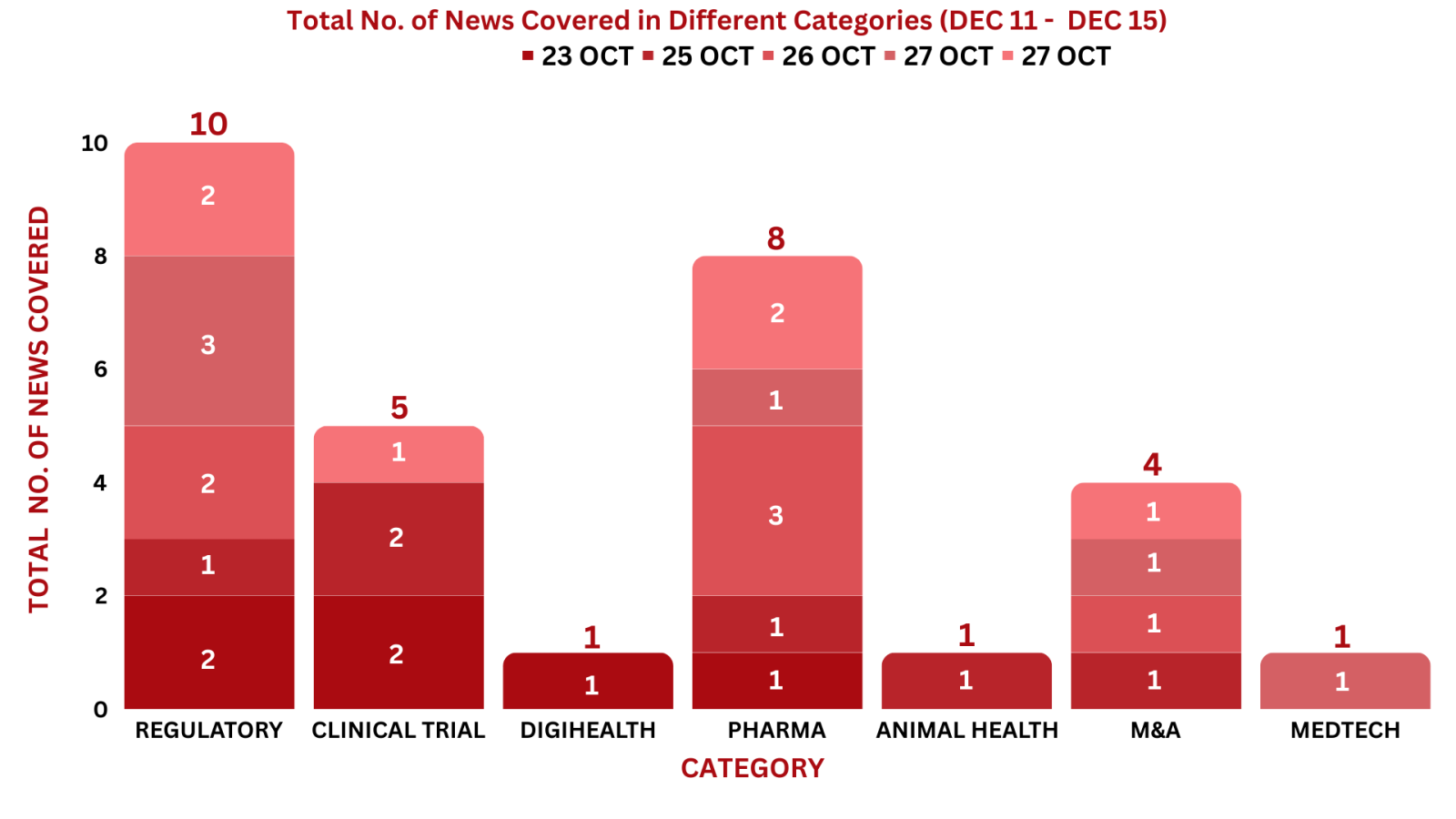
PharmaShots Weekly Snapshots (December 11 – December 15, 2023)
This week PharmaShots’ news was all about the updates on regulatory, Clinical Trials, Digihealth, Pharma, Animal Health, M&A & MedTech. Check out our full report below:

The EC Grants Conditional Marketing Authorisation to Pfizer’s Elerexfio for the treatment of Relapsed and Refractory Multiple Myeloma
Read More: Pfizer
The US FDA Approves Basilea’s Cresemba for the Treatment of Invasive Aspergillosis & Invasive Mucormycosis in Children
Read More: Basilea
GSK's Regulatory Application for Arexvy to Prevent RSV-Related Diseases has been Accepted by MHLW
Read More: GSK
The NMPA Accepts NDA Application for Henlius’s Hansizhuang for the treatment of Esophageal cancer
Read More: Henlius
The EC Approves Blueprint Medicines’s Ayvakyt for the Treatment of Indolent Systemic Mastocytosis (ISM)
Read More: Blueprint Medicines
Amgen Receives the US FDA’s Acceptance on its BLA for Tarlatamab with a Priority Review Grant for Advanced Small Cell Lung Cancer
Read More: Amgen
Abbisko Therapeutics’ Pimicotinib (ABSK021) has Obtained US FDA’s FTD for Treating Tenosynovial Giant Cell Tumor
Read More: Abbisko Therapeutics
The US FDA to Form a New Advisory Committee to Evaluate Genetic Metabolic Disease Treatments
Read More: US FDA
The US FDA Approves Merck & Co.’s Welireg (belzutifan) for the Treatment of Advanced Renal Cell Carcinoma (RCC)
Read More: Merck
The US FDA Approves Glaukos’ iDose TR (travoprost intracameral implant) for Ocular Hypertension (OHT) or Open-Angle Glaucoma (OAG) Patients
Read More: Glaukos

Novartis Reports Favorable Outcomes for Iptacopan in P-III (APPEAR-C3G) for C3 Glomerulopathy Treatment (C3G)
Read More: Novartis
Sanofi Highlights the P-III Results for Sarclisa (isatuximab)+ KRd to Treat Multiple Myeloma
Read More: Sanofi
Nurix Highlights Favorable BTK degraders, NX-5948 and NX-2127 in P-Ia/Ib for Lymphoma
Read More: Nurix
Regeneron reveals Favorable outcomes of Odronextamab in P-II (ELM-2) Trial for the Treatment of Follicular Lymphoma
Read More: Regeneron
Moderna and Merck Reveal Results from the P-IIb (KEYNOTE-942) Trial of mRNA-4157 (V940) + Keytruda (pembrolizumab) for Resected High-Risk Melanoma
Read More: Moderna & Merck

Medtronic Expands collaboration with Cosmo Pharmaceuticals, leveraging advanced AI for transformative endoscopy
Read More: Medtronic & Cosmo Pharmaceuticals

MD Anderson & Rigel Pharmaceuticals unveil a strategic collaboration to progress the development of olutasidenib
Read More: MD Anderson & Rigel Pharmaceuticals
BMS has entered into an exclusive license agreement with Systimmune for the development of BL-B01D1
Read More: BMS
Hutchmed Completes Enrollment for P-II/III Trial of Fruquintinib + Sintilimab for Renal Cell Carcinoma in China
Read More: Hutchmed
Eisai announces the launch of Leqembi (Lecanemab) for the Treatment of Alzheimer's Disease (AD)
Read More: Eisai
ADC Therapeutics Reveals the Results of Zynlonta in P-II for Follicular Lymphoma
Read More: ADC Therapeutics
Nerviano Medical and Italfarmaco Collaborate to Develop and Commercialize Peptide-Drug Conjugate (PDC)
Read More: Nerviano Medical & Italfarmaco
Nona Biosciences Enters into a License Agreement with Pfizer to Develop and Commercialize HBM9033 for Solid Tumors
Read More: Nona Biosciences & Pfizer
Terray Therapeutics and BMS Partner for the Discovery and Development of Small Molecules
Read More: Terray Therapeutics & BMS

The EMA’s CVMP Adopts PositiveOpinion for Merck’s Bravecto to Protect Dogs Against Ticks and Fleas
Read More: Merck

Imbio Acquired by 4DMedical for the Amount of ~$45M
Read More: Imbio
AstraZeneca announces the Acquisition of Icosavax for ~$1.1B
Read More: AstraZeneca
For about $280M, Integra LifeSciences to Acquire Acclarent
Read More: Integra LifeSciences & Acclarent
With Hopes to Enhance its Animal Health Business, Zenex Acquires Ayurvet
Read More: Zenex & Ayurvet

The US FDA’s Approves, Medtronic’s PulseSelect PFA System for the Treatment of Atrial Fibrillation
Read More: Medtronic
Related Post:- PharmaShots Weekly Snapshots (December 04 – December 08, 2023)
Tags

Kritika is a content writer at PharmaShots. She is interested in covering recent innovations from the pharma & MedTech industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.














